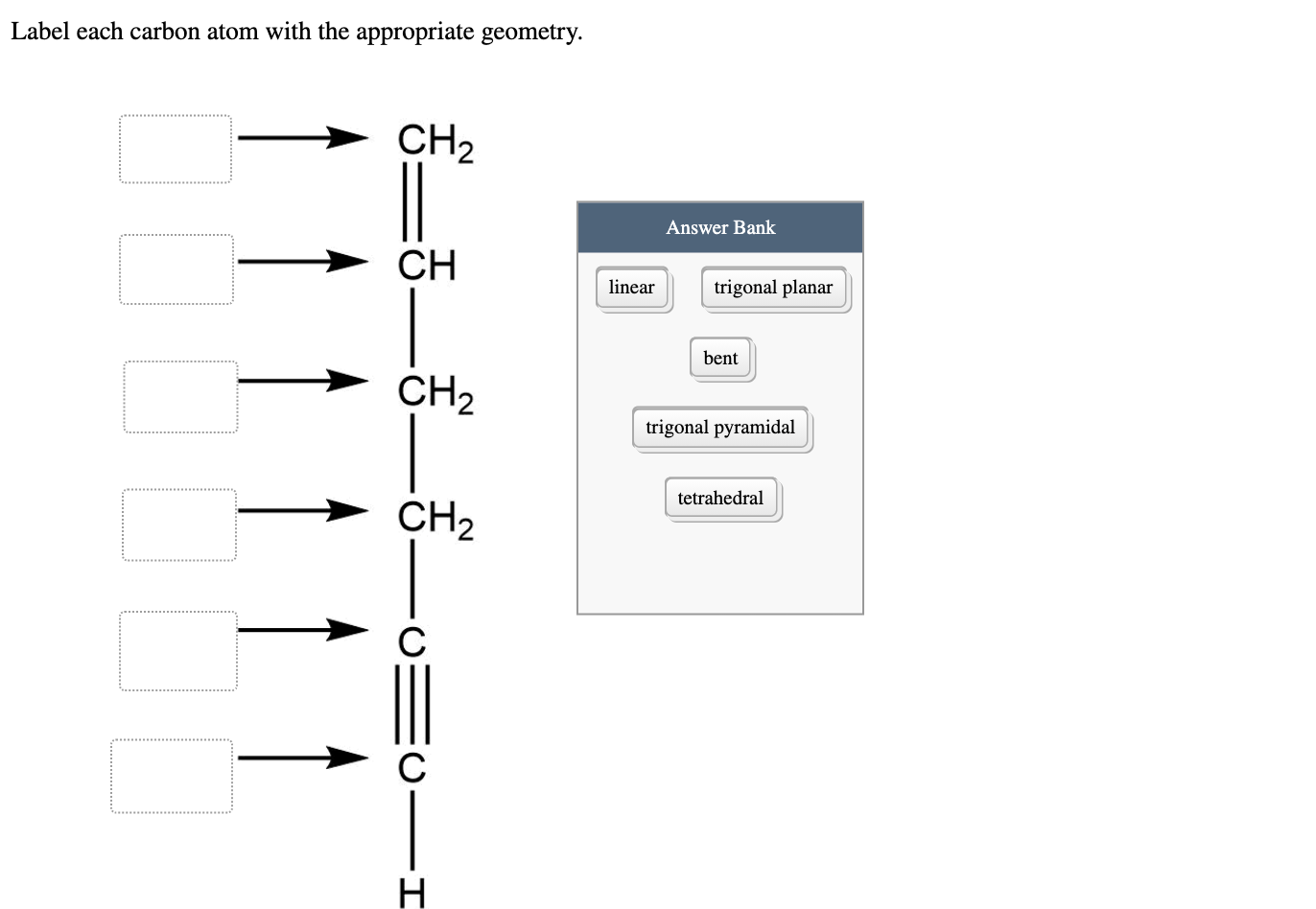
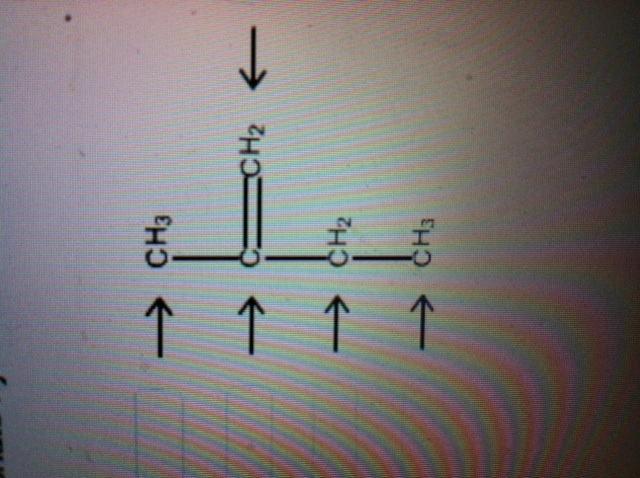
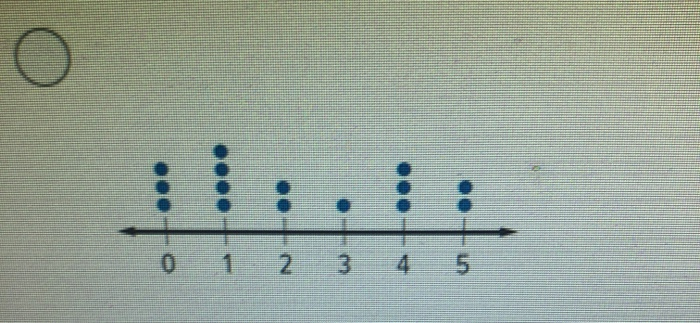
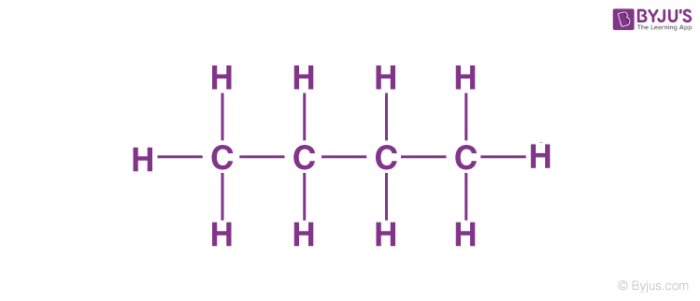
39 label each carbon atom with the appropriate geometry
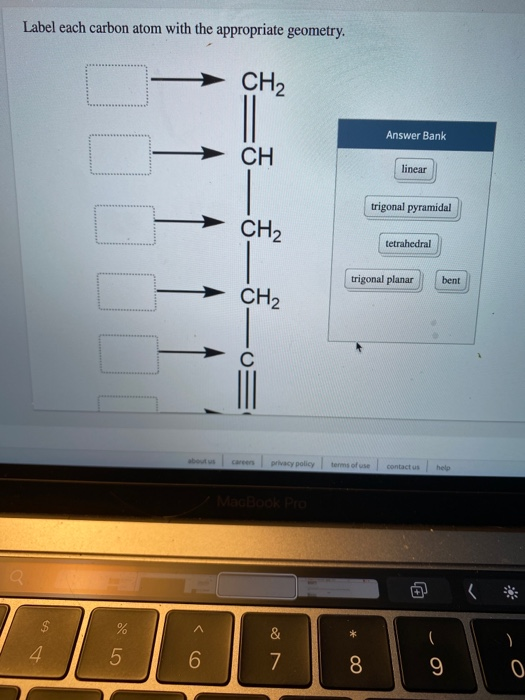
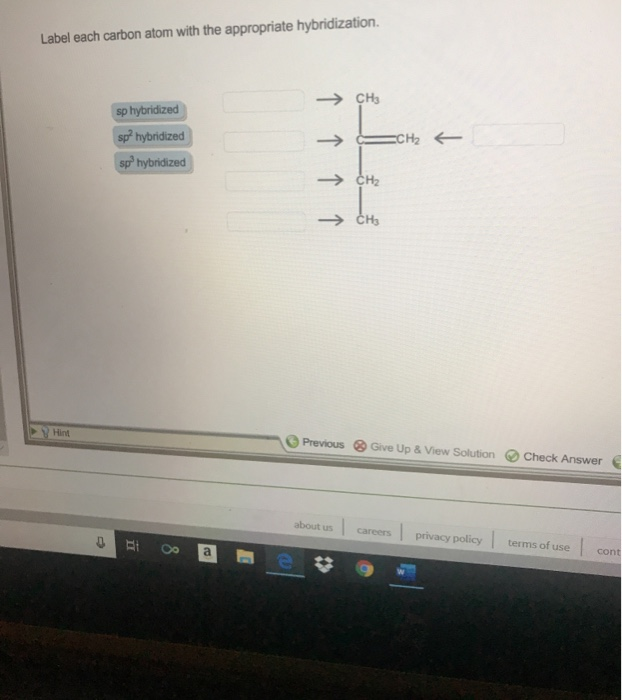
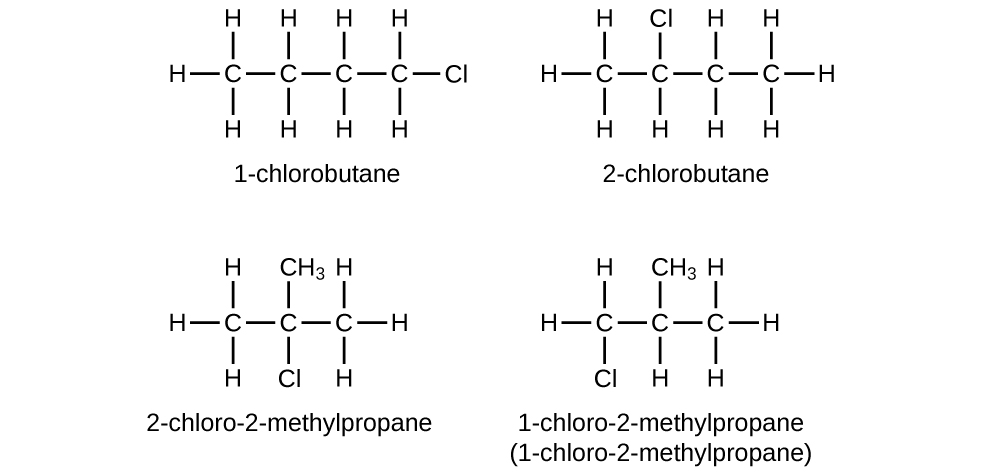
label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... E-Z notation for geometric isomerism - chemguide The atoms attached directly to the carbon of the CH 2 group are C H H. In the second list, the C is written first because it has the highest atomic number. Now compare the two lists atom by atom. The first atom in each list is an H in the CH 3 group and a C in the CH 3 CH 2 group. The carbon has the higher priority because it has the higher ...
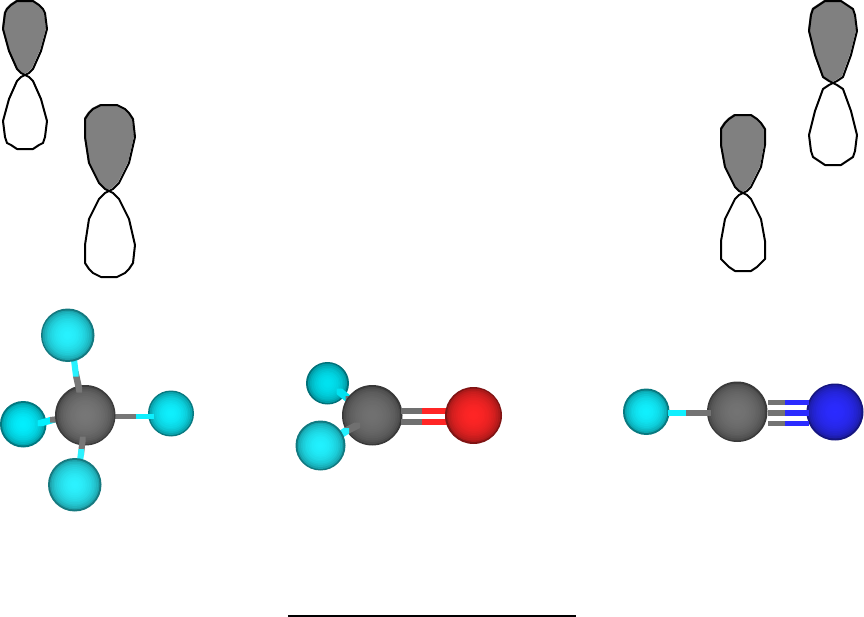
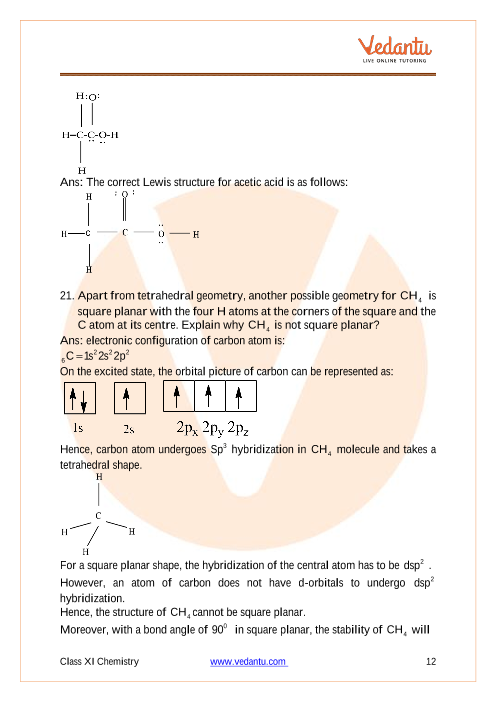
Molecular Geometry - Oklahoma State University-Stillwater For trigonal pyramidal geometry the bond angle is slightly less than 109.5 degrees, around 107 degrees. For bent molecular geometry when the electron-pair geometry is tetrahedral the bond angle is around 105 degrees. Lets consider the Lewis structure for CCl 4. We can draw the Lewis structure on a sheet of paper.
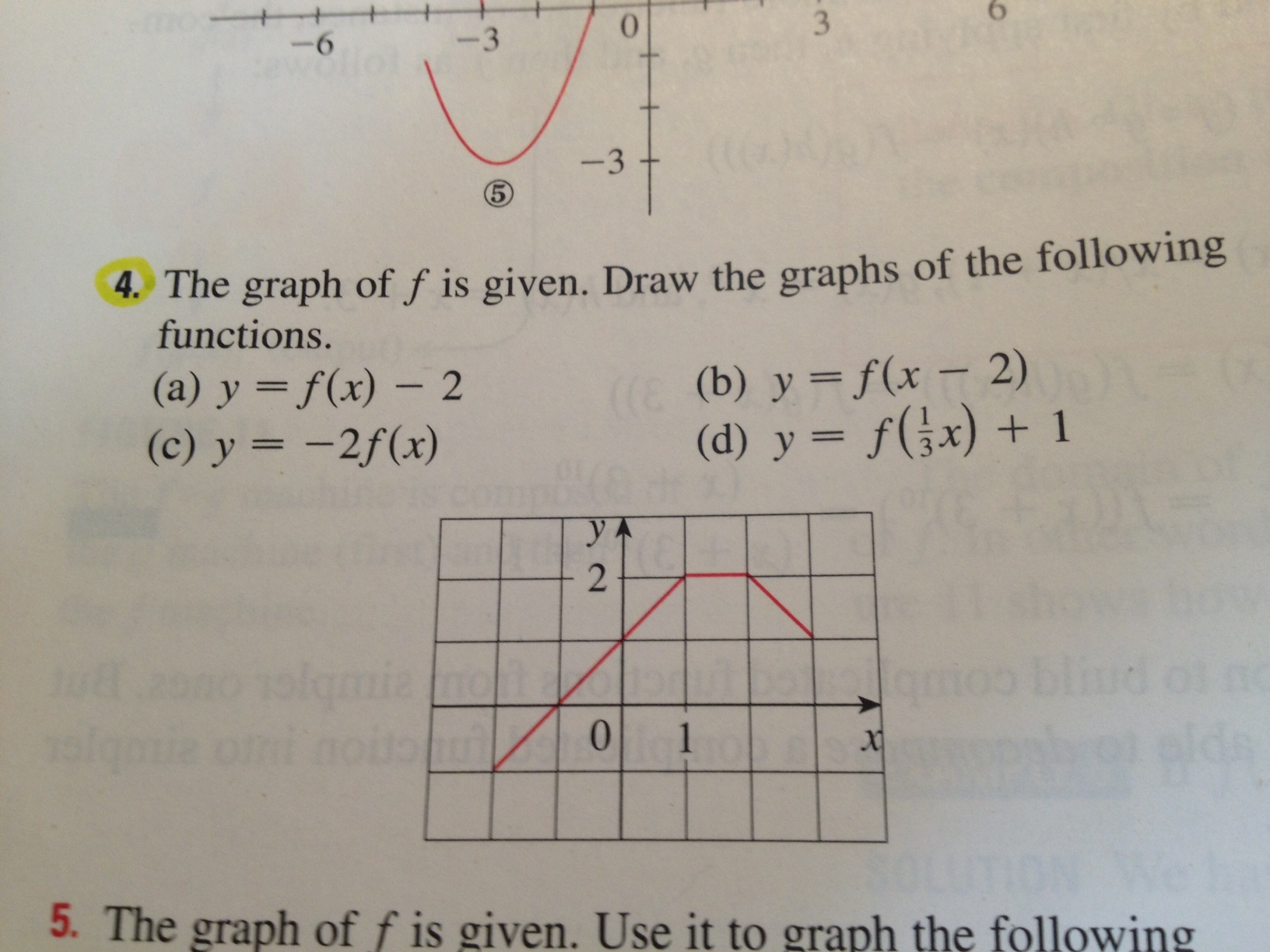
Label each carbon atom with the appropriate geometry
The Structure of an Atom Explained With a Labeled Diagram Basic Diagram of an Atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. When one says an atom is electrically neutral, it means that the number ... Finding the hybridization of atoms in organic molecules ... - Khan Academy Worked examples: Finding the hybridization of atoms in organic molecules. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Label each carbon atom with the appropriate geometry. Answer to Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ČH2 tetrahedral bent tetrahedra | SolutionInn
Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate geometry. - Transtutors Label each carbon atom with the appropriate geometry. Trigonal pyrimidal Trigonal planar Tetrahedral Linear Bent CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (single bond) C (triple bond) CH (Get Answer) - Label each carbon atom with the appropriate geometry ... Label each carbon atom with the appropriate geometry. Bent trigonal pyramidal trigonal planar tetrahedral linear Label each carbon atom with the appropriate geometry. - OneClass 5 Nov 2019. Label each carbon atom with the appropriate geometry. Trigonal pyrimidal. Trigonal planar. Tetrahedral. Linear. Bent. CH2 (double bond) CH (single bond) CH2 (single bond) CH2 (singlebond) C (triple bond) CH. Show full question. Solved Label each carbon atom with the appropriate geometry. - Chegg This problem has been solved! Label each carbon atom with the appropriate geometry. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Label each carbon atom with the appropriate geometry.
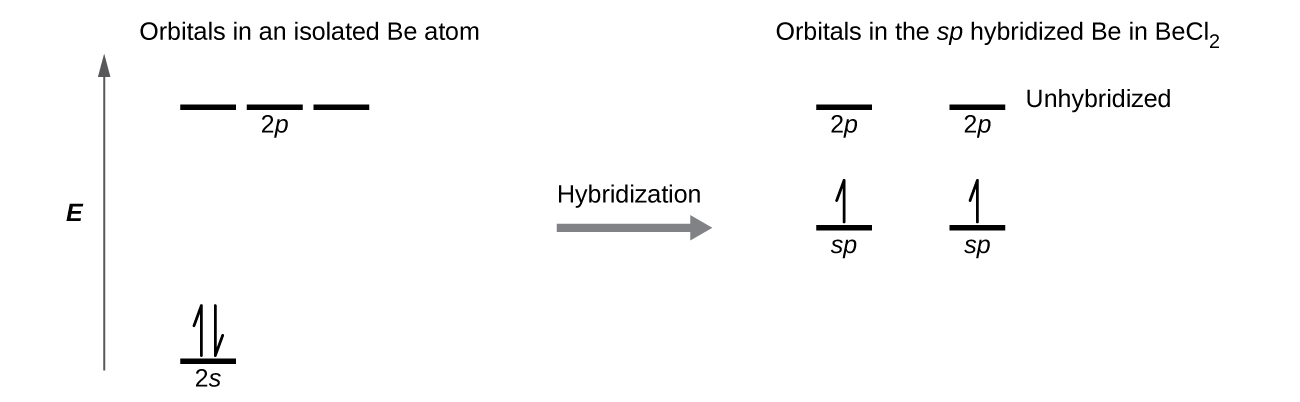
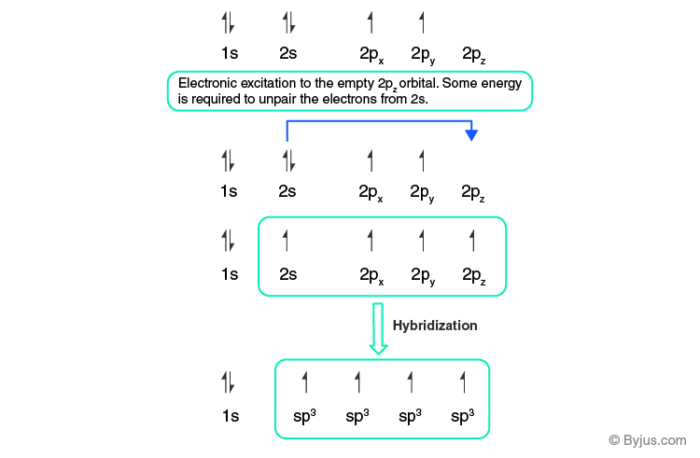
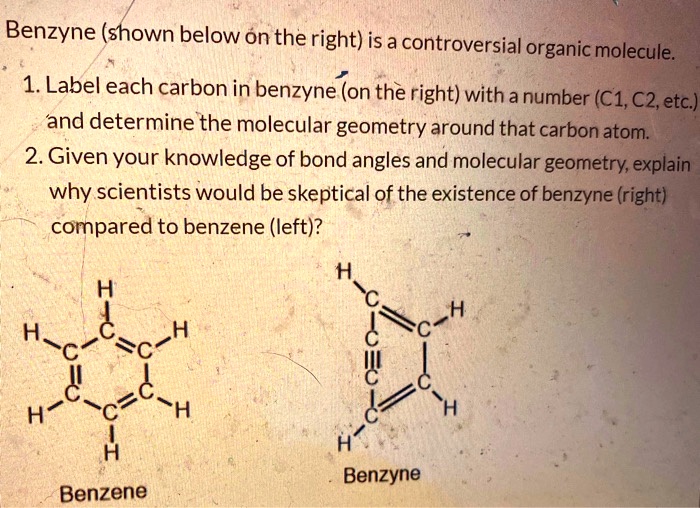
Carbon dioxide (CO2) lewis dot structure, molecular geometry, bond ... The bond angle of CO2. CO2 has a bond angle of 180º. In CO2, the carbon (C) central atom has no lone pair and is attached to two oxygen (O) atoms. Therefore, no distortion occurs around the central atom which makes it linear in shape that has a bond angle of 180º. Watch this video on YouTube. Hybridization of Carbon - Molecular Geometry and Bond Angles A carbon atom is sp2 hybridized when bonding takes place between 1 s-orbital with two p orbitals. There is a formation of two single bonds and one double bond between three atoms. The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite 3. sp3 Hybridization What is the geometry around each of the three central atoms in the CH ... Explanation: We must first draw the Lewis structure of acetic acid. (Adapted from Chemistry@TutorVista.com) Carbon 1 This atom has four atoms directly attached and no lone pairs. Its electron geometry and its molecular geometry are both tetrahedral as in methane. Carbon 2 This atom has three atoms directly attached and no lone pairs. ⚗️Label each carbon atom with the appropriate geometry. Bin 1 points to ... Label each carbon atom with the appropriate geometry. Bin 1 points to a carbon bonded to a double bonded carbon and single bonded to two hydrogens. Bin 2 points to a carbon double bonded to a carbon and single bonded to a carbon and one hydrogen. Bin 3 is a carbon single bonded to two carbons and single bonded to two hydrogens.
Label each carbon atom with the appropriate geometry ... - Course Hero The hybrid orbitals of carbon involve in bond formation with hydrogen. Hence, the geometry at each carbon depends on the type of hybridization. Fundamentals The geometry of sp3 hybridized carbon atom is tetrahedral. The geometry of sp2 the hybridized carbon atom is trigonal planar. The geometry of sp hybridized carbon atom is linear. Molecular Geometry - Chem1 Tetrahedrally-coordinated carbon chains. Carbon atoms are well known for their tendency to link together to form the millions of organic molecules that are known. We can work out the simpler hydrocarbon chains by looking at each central atom separately. Thus the hydrocarbon ethane is essentially two CH 3 tetrahedra joined end-to-end. Chapter 9 Homework Flashcards - Questions and Answers | Quizlet There are 6 C atoms in the molecule. Starting on the left, the hybridizations are: sp2, sp2, sp3, sp, sp, sp3. All single bonds are bonds. Double and triple bonds each contain 1 bond. This molecule has 8 C-H bonds and 5 C-C bonds, for a total of 13 bonds. Double bonds have 1 bond and triple bonds have 2 bonds. This molecule has a total of 3 bonds. OChem Spring 2017 Exam 1 Flashcards - Quizlet Label each carbon atom wiht the appropriate geometry (cover right side) Sapling Hw Ch 1.21 Predict the molecular shape of methane, the carbonate ion, carbon dioxide, and the sulfite ion (cover left side of screen)
CO2 Lewis Structure, Molecular Geometry and Hybridization CO2 Molecular Geometry. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other.
Absolute Configuration Rules & Examples - Study.com R and S Configuration. To name stereoisomers with different absolute configurations, each chiral center is given a label of R- or S-. A chiral center is an atom (usually carbon) that has four ...
Answered: Label each carbon atom with the… | bartleby Solution for Label each carbon atom with the appropriate geometry. CH2 Answer Bank CH tetrahedral linear trigonal planar bent CH2 trigonal pyramidal CH2 C C H.
7.6 Molecular Structure and Polarity - Chemistry Predict the local geometry for the nitrogen atom, the two carbon atoms, and the oxygen atom with a hydrogen atom attached: Solution. Consider each central atom independently. The electron-pair geometries: nitrogen--four regions of electron density; tetrahedral; carbon (CH 2)--four regions of electron density; tetrahedral
Solved Label each carbon atom with the appropriate | Chegg.com Chemistry. Chemistry questions and answers. Label each carbon atom with the appropriate hybridization. sp,sp2 or sp3.
How to Identify Chiral Carbons | Identify Chiral Carbons ... - Pediaa.Com Step 1. First, determine whether the groups attached to the carbon atom are different from each other. If they are different, we can guess that it as a chiral carbon. In the above image, the molecule has a hydrogen atom and a methyl group attached to the same carbon atom. But other two groups have formed a ring.
Quiz1 88%.docx - Question 1 Label each carbon atom with the appropriate ... Question 1 Label each carbon atom with the appropriatehybridization. Question 2 Determine the formal charge on each atom in thestructure. Question 3 Predict the approximate bond angles in the molecule. Question 4 Given six molecules, identify the molecules with polar bonds and the molecules that arepolar. a) Which molecules have polarbonds?
Label each carbon atom with the appropriate geometry. Answer to Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ČH2 tetrahedral bent tetrahedra | SolutionInn
Finding the hybridization of atoms in organic molecules ... - Khan Academy Worked examples: Finding the hybridization of atoms in organic molecules. We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules.
The Structure of an Atom Explained With a Labeled Diagram Basic Diagram of an Atom. Most of an atom is just empty space and consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The center of an atom is the nucleus and one or more electrons surrounding the nucleus. When one says an atom is electrically neutral, it means that the number ...






















Post a Comment for "39 label each carbon atom with the appropriate geometry"