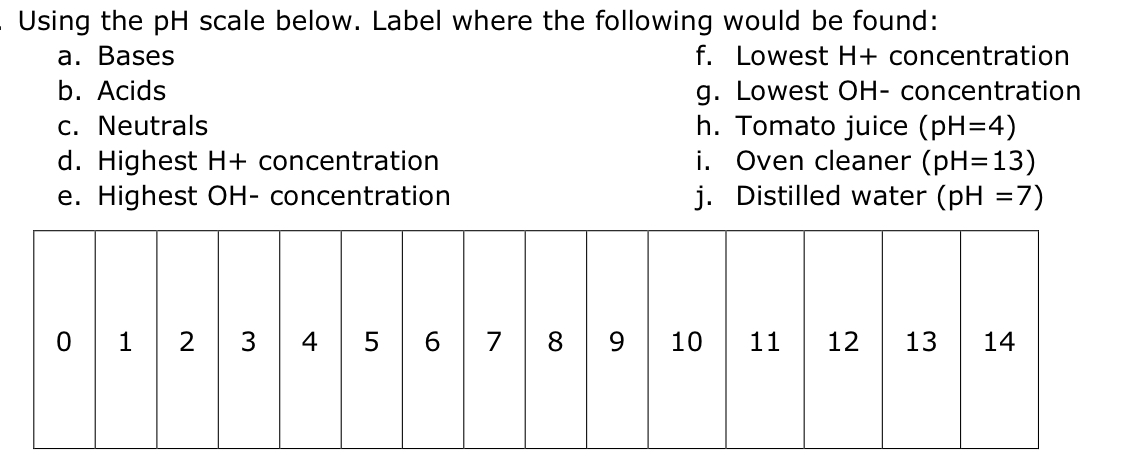
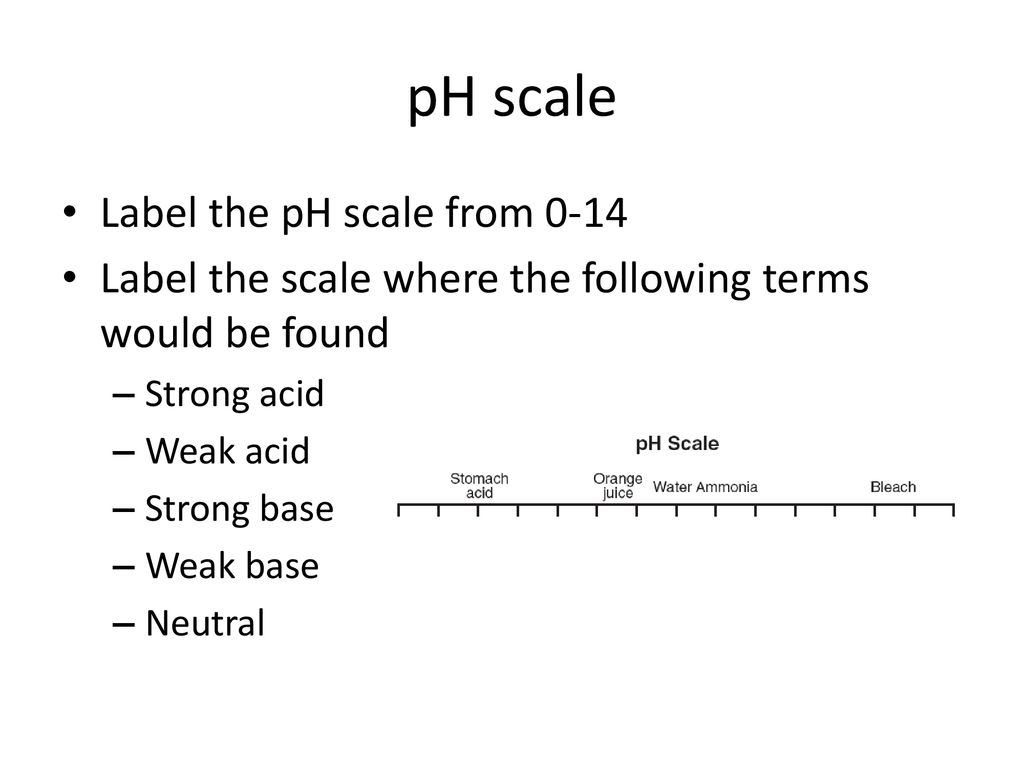
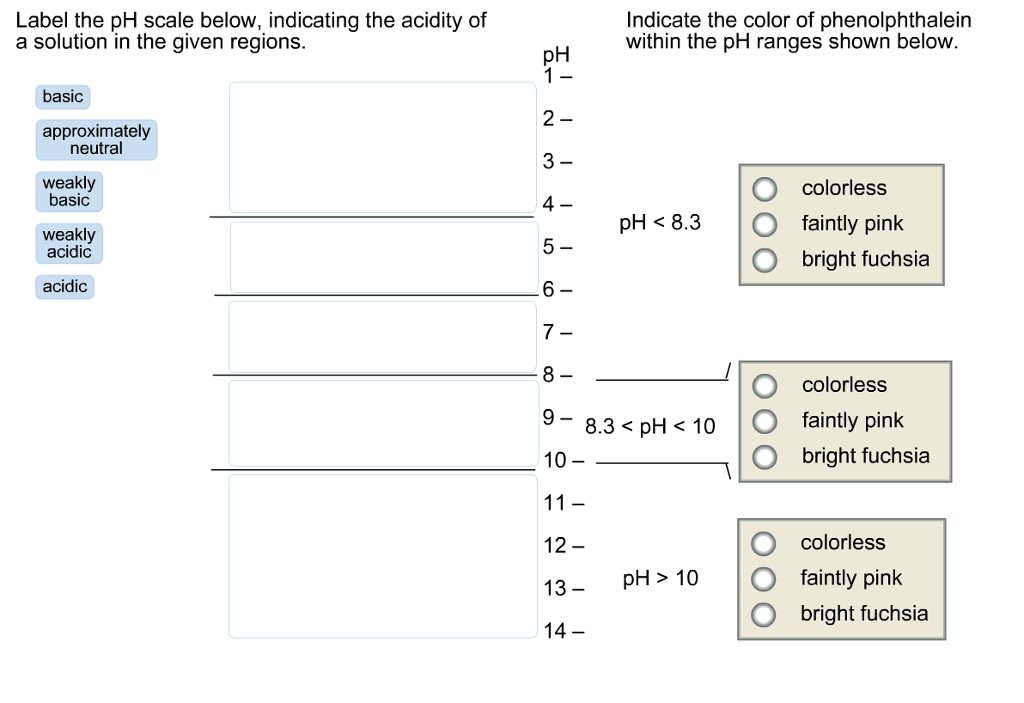
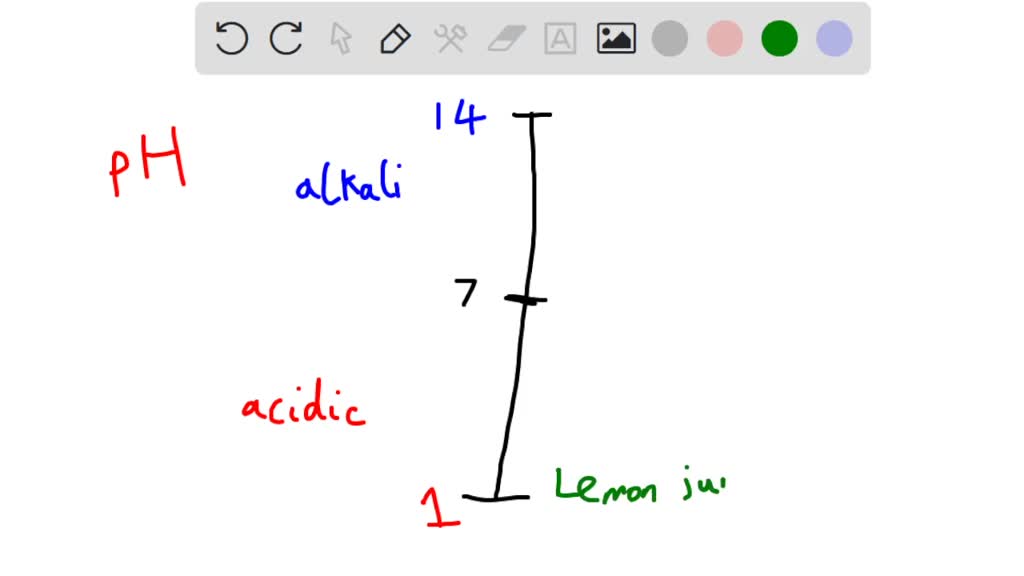
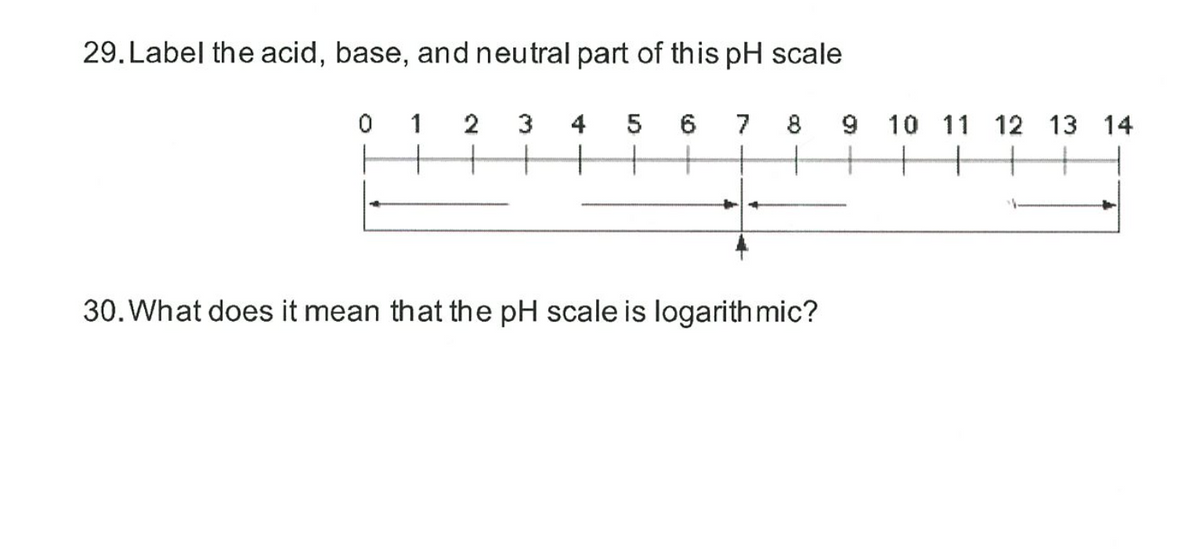
44 label the ph scale
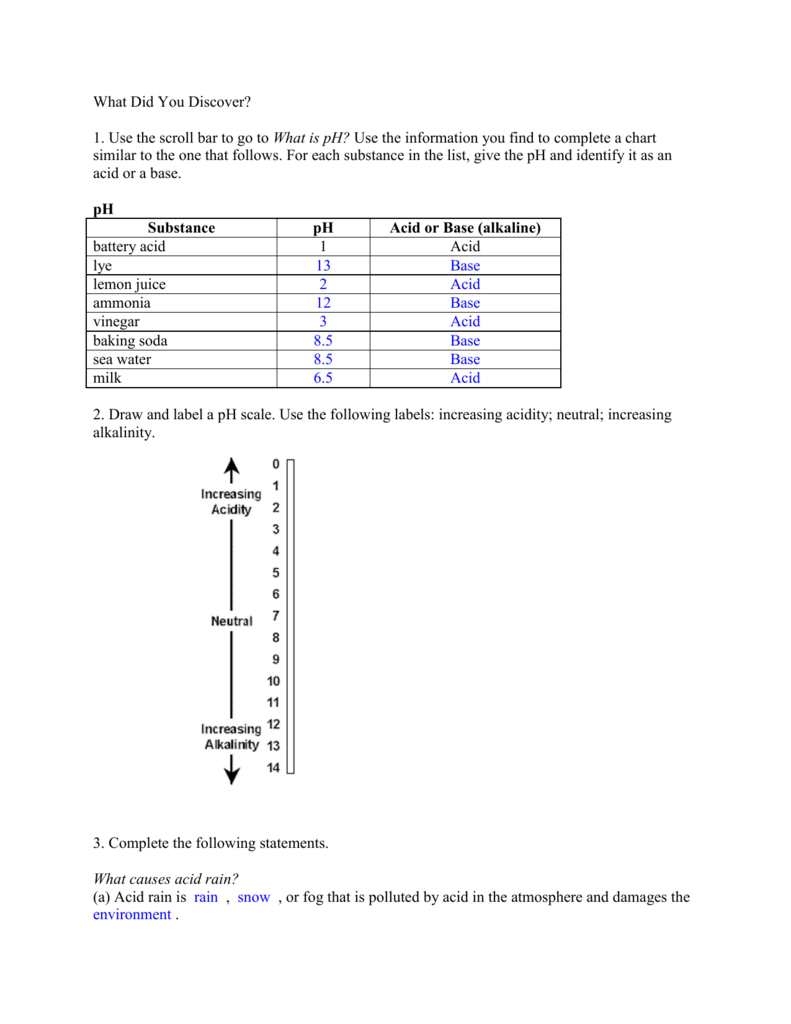
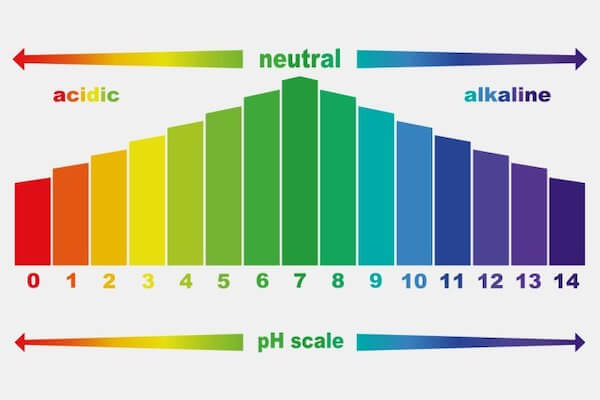
pH Scale | U.S. Geological Survey Jun 19, 2019 · pH is a measure of how acidic/basic water is. The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that ... 6.3: The pH Scale - Mathematics LibreTexts Sep 12, 2020 · This is known as the pH scale. You can use pH to make a quick determination of whether a given water based solution is acidic, basic, or neutral. Example Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5 pure water, pH = 7 wine, pH = 3.0 Solution
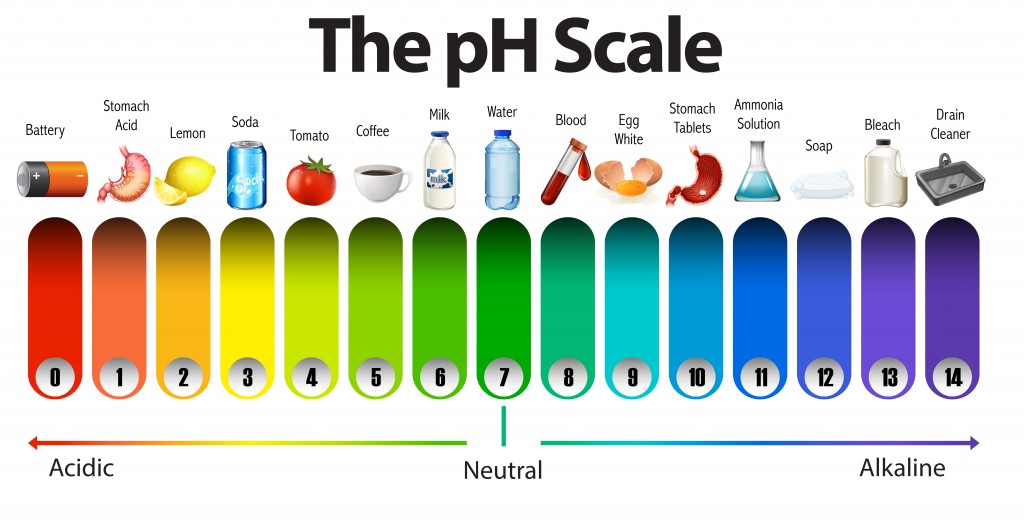
The pH Scale | Biology for Majors I - Lumen Learning The pH scale is, as previously mentioned, an inverse logarithm and ranges from 0 to 14 (Figure 1). Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Extremes in pH in either direction from 7.0 are usually considered inhospitable to life.
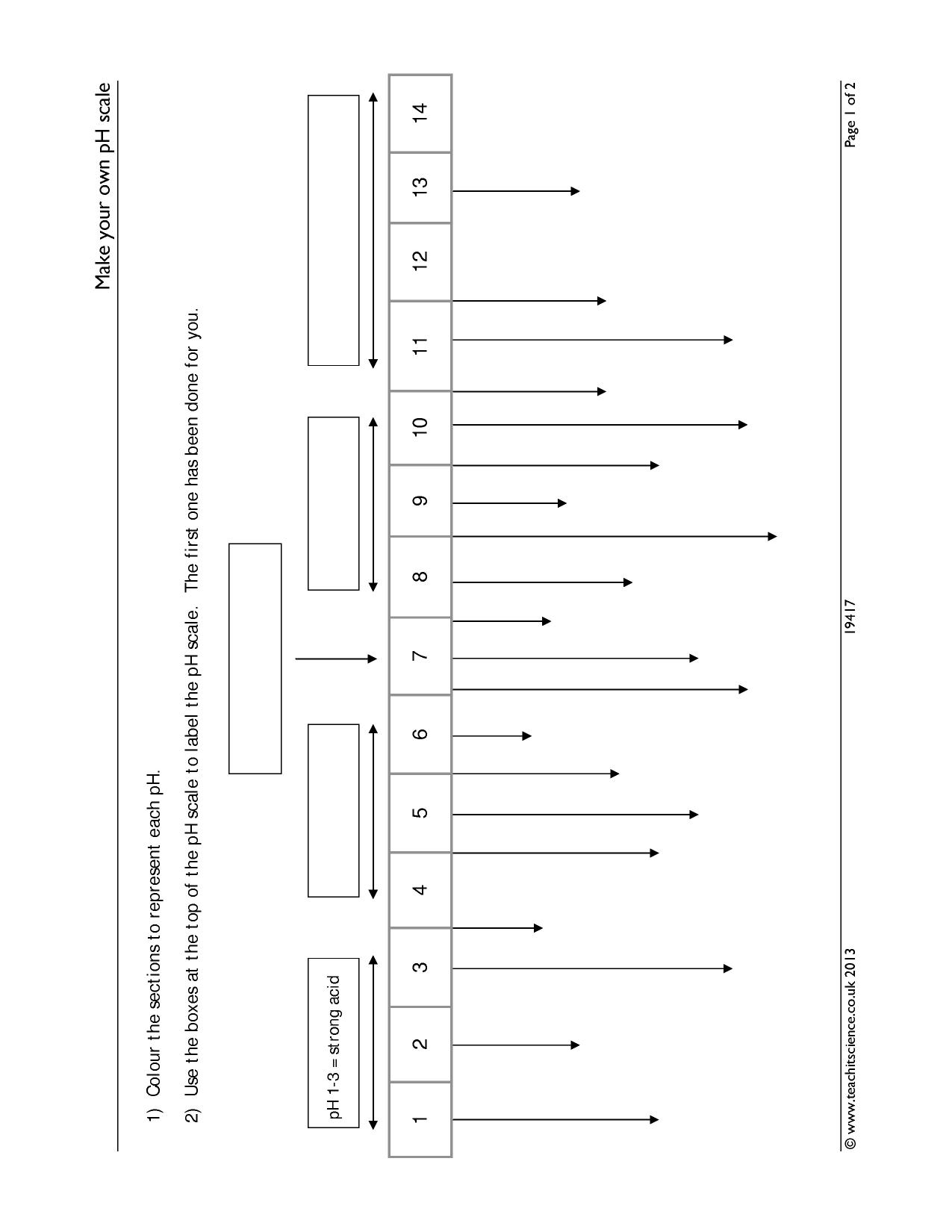
Label the ph scale
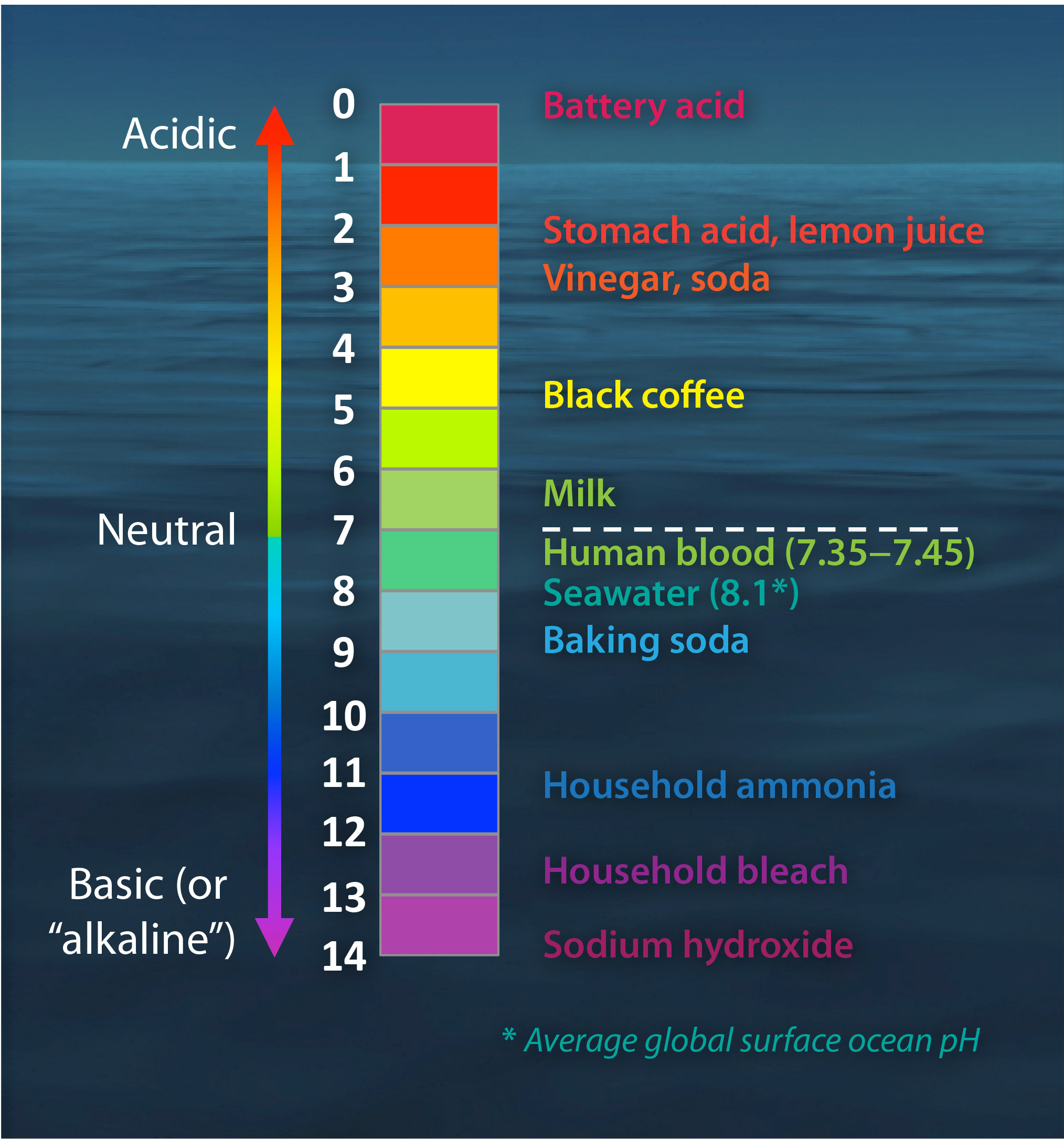
Acids, Alkalis, and the pH Scale – Compound Interest Jul 9, 2015 · A pH of spot on 7 denotes a neutral solution (neither acidic or alkaline). Any pH below 7 is acidic, whilst any pH above 7 is termed alkaline. Water molecules have the chemical formula H 2 O. However, these molecules are capable of splitting up slightly in solution, in H + and OH – (hydroxide) ions. Draw neat and labeled diagram of pH scale? - Toppr Ask The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Similar questions What colour does Universal Indicator go in a liquid with a pH of 9? Medium View solution > pH Scale Flashcards | Quizlet pH Scale a scale used to define the levels of hydrogen (H) or hydroxide (OH) ions in a solution. Ranges from 0 (acidic) to 14 (basic/alkaline), with 7 being neutral. Hydrogen Ion an ion of hydrogen (H) created by the breaking up of a water molecule during the mixing of a solution. (Negatively charged) Hydroxide Ion
Label the ph scale. pH Scale | U.S. Geological Survey The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. Normal, clean rain has a pH value of between 5.0 and 5.5, which is slightly acidic. pH Scale Flashcards | Quizlet pH Scale a scale used to define the levels of hydrogen (H) or hydroxide (OH) ions in a solution. Ranges from 0 (acidic) to 14 (basic/alkaline), with 7 being neutral. Hydrogen Ion an ion of hydrogen (H) created by the breaking up of a water molecule during the mixing of a solution. (Negatively charged) Hydroxide Ion Draw neat and labeled diagram of pH scale? - Toppr Ask The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Similar questions What colour does Universal Indicator go in a liquid with a pH of 9? Medium View solution > Acids, Alkalis, and the pH Scale – Compound Interest Jul 9, 2015 · A pH of spot on 7 denotes a neutral solution (neither acidic or alkaline). Any pH below 7 is acidic, whilst any pH above 7 is termed alkaline. Water molecules have the chemical formula H 2 O. However, these molecules are capable of splitting up slightly in solution, in H + and OH – (hydroxide) ions.

























Post a Comment for "44 label the ph scale"